General Areas of Expertise
ORGANOIDS
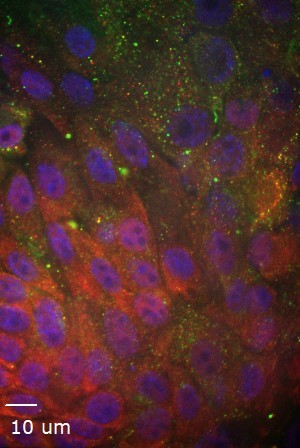
Organoids are three-dimensional (3D) cell cultures generated from stem or organ progenitor cells that self-organize and can remain both genetically and phenotypically stable throughout long-term culture (months to >1 year, depending on the tissue of origin) and therefore offer a unique and novel culture model that bridges the gap between in vitro cell culture and in vivo animal studies of reproductive physiology and pathology. Importantly, organoids reduce and in some cases eliminate the need for research animals and improve animal welfare which is important to our lab.
EXTRACELLULAR VESICLES
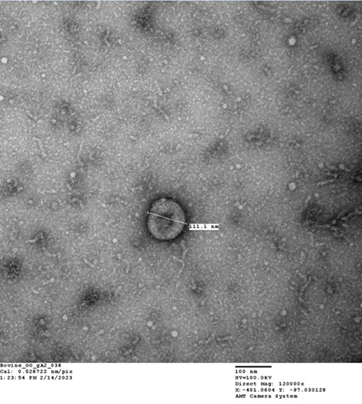
Extracellular Vesicles or ‘EVs’ are a heterogenous group of membranous nanoparticles that are secreted by all cells.. EVs induce changes in the target cell via cell-to-cell transfer of their biologically active cargos consisting of functional proteins, lipids, mRNA and miRNAs. As natures very own nanoparticles, EVs are inherently stable, biocompatible, immune tolerant, able to cross biological barriers and be taken up by cells in target organs. EVs therefore hold great promise as an alternative vehicle to cell based therapeutics and current contraceptive agents.
MESENCHYMAL STEM CELL CULTURE
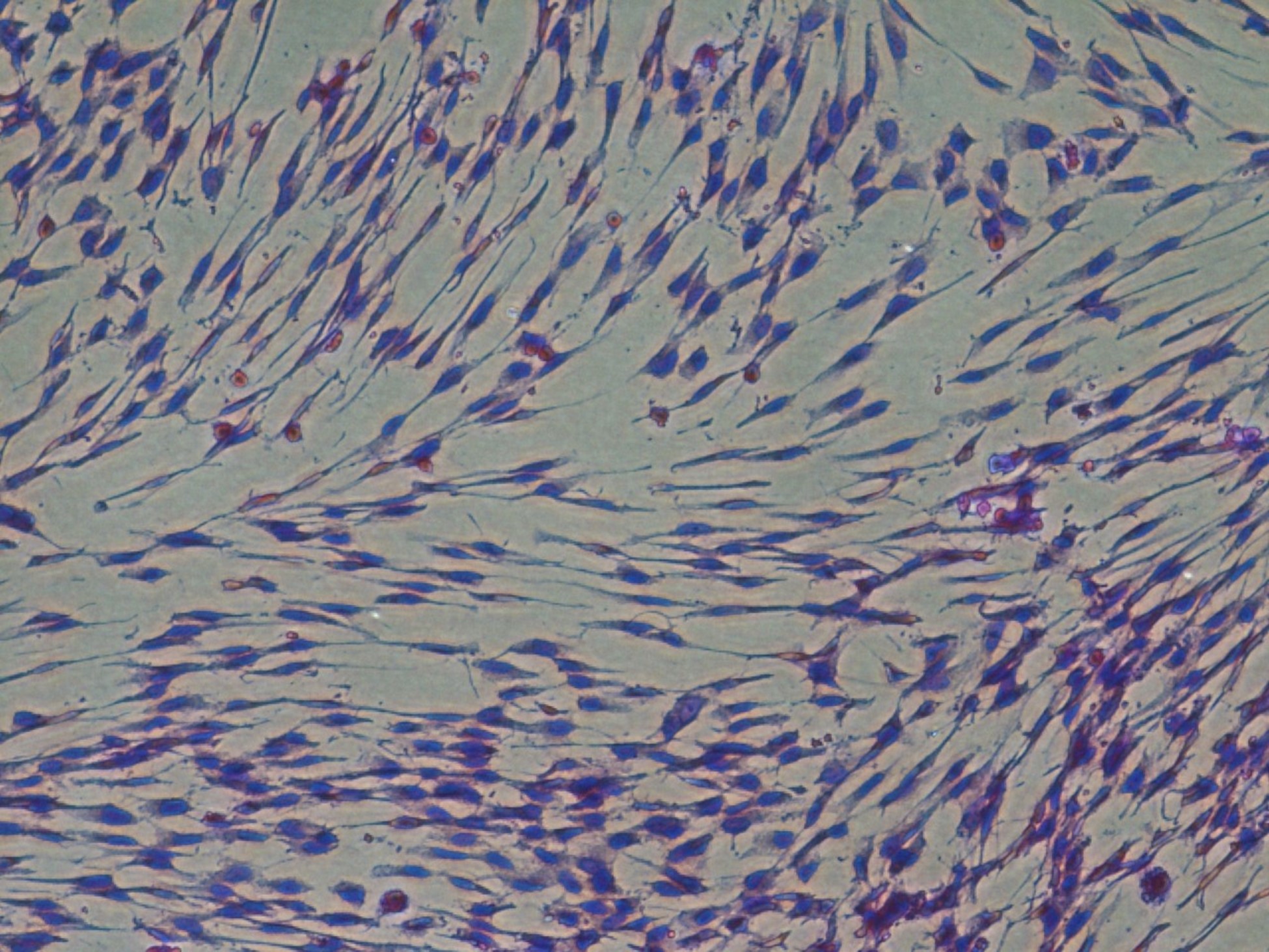 Our lab has established lines of canine and equine early gestational MSC. We investigate the use of these novel MSCs and their extracellular vesicles as potential therapeutics for a number of diseases including equine post breeding induced endometritis, canine endometritis and osteoarthritis in horses.
Our lab has established lines of canine and equine early gestational MSC. We investigate the use of these novel MSCs and their extracellular vesicles as potential therapeutics for a number of diseases including equine post breeding induced endometritis, canine endometritis and osteoarthritis in horses.
SEMEN LAB
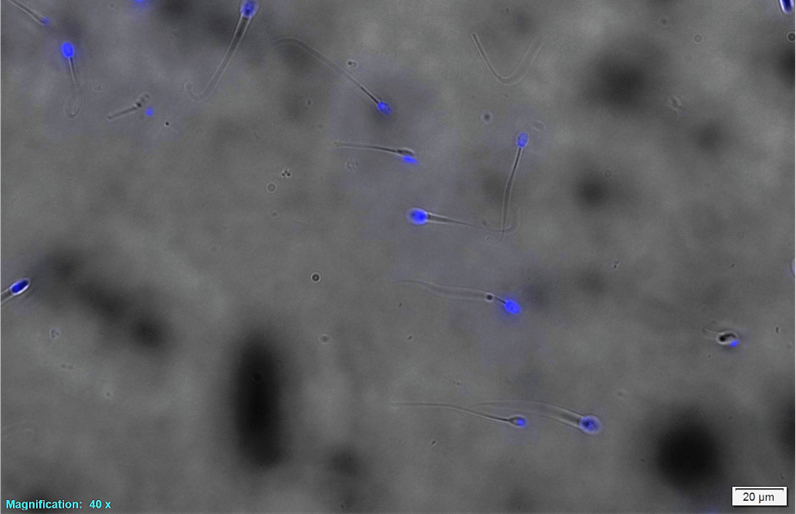
Our lab has investigated the addition of different cryo-protectants to freezing extender to optimize the post thaw viability of canine semen. We are working with Sexing Technologies to optimize the sexing of canine semen using specialized flow cytometry technology and develop methods and specialized canine semen extenders to successful cryopreserve sex sorted canine sperm. We are also investigating the effects of extracellular vesicles derived from prostatic fluid on capacitation, viability and longevity after addition to canine semen after thawing.
Current Research Projects

Equine chorionic girdle (CG) organoids and their in vitro production of equine chronic gonadotropin (eCG) (bioreactor)
The equine chorionic girdle is a unique placental structure in the mare that is composed of specialized invasive trophoblast cells that will form endometrial cups and secrete the glycoprotein hormone equine chorionic gonadotropin (eCG), also known as pregnant mare serum gonadotropin (PMSG). In addition to its importance in pregnancy maintenance in the mare, eCG administration is essential for many domestic and non-domestic animal breeding programs which include cattle, sheep, goats, pigs, cats, and captive wildlife species. However, horse welfare is negatively impacted to produce this hormone commercially. Our lab has successfully generated equine chorionic girdle organoids that produce eCG and develop an ultrastructure that is characteristic of in vivo equine endometrial cups. These organoids will be cultured with a spinning bioreactor system to determine if eCG production can be increased. Furthermore, we are exploring the utilization of this model for studying early pregnancy in the mare.
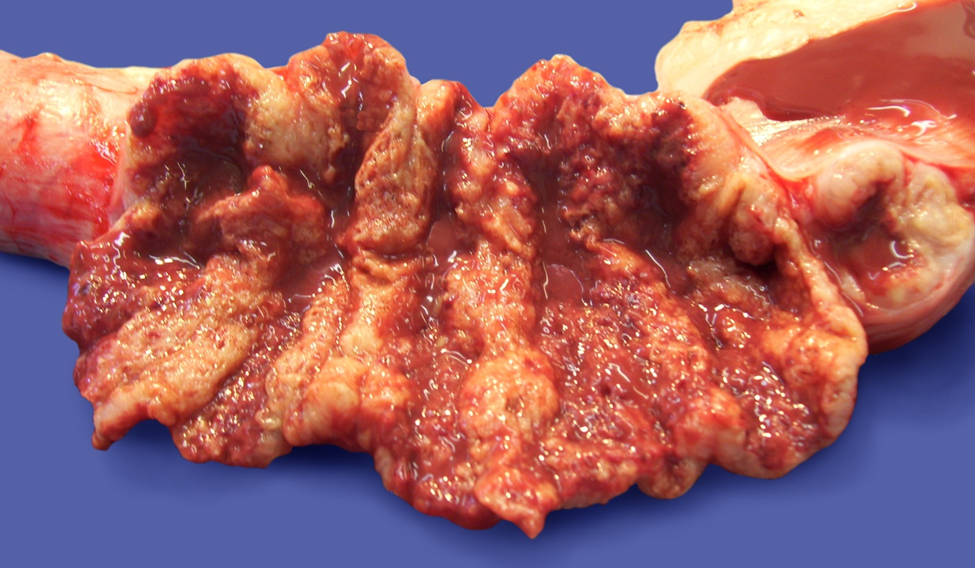
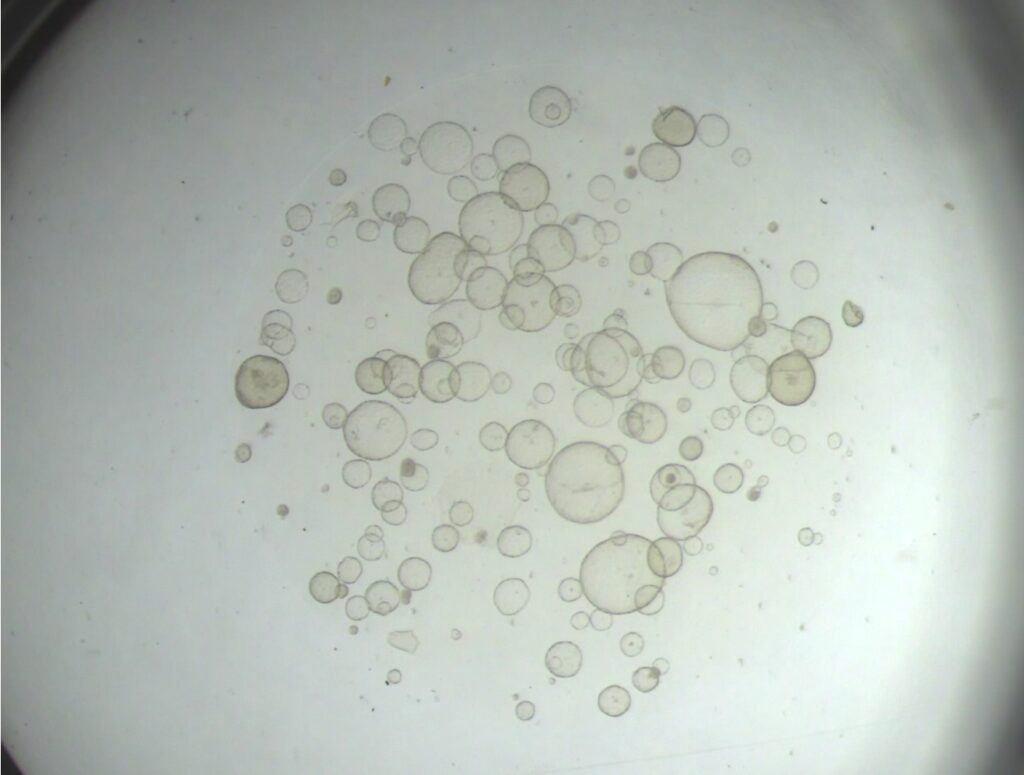
Canine endometrial organoids as a model to study endometrial disease and investigate therapeutics
Canine endometrial organoids are used to enhance our understanding of common reproductive pathologies in the bitch without the need for research animals. Some of the pathologies being studied using this model include endometritis, cystic endometrial hyperplasia (CEH), and life-threatening pyometra, which is estimated to affect >66% of intact bitches over 9 years of age. A greater understanding of these endometrial pathologies will lead to improved health and welfare of intact bitches, enhanced fertility in domestic and wild canine breeding populations, and increased understanding of canine reproductive diseases.
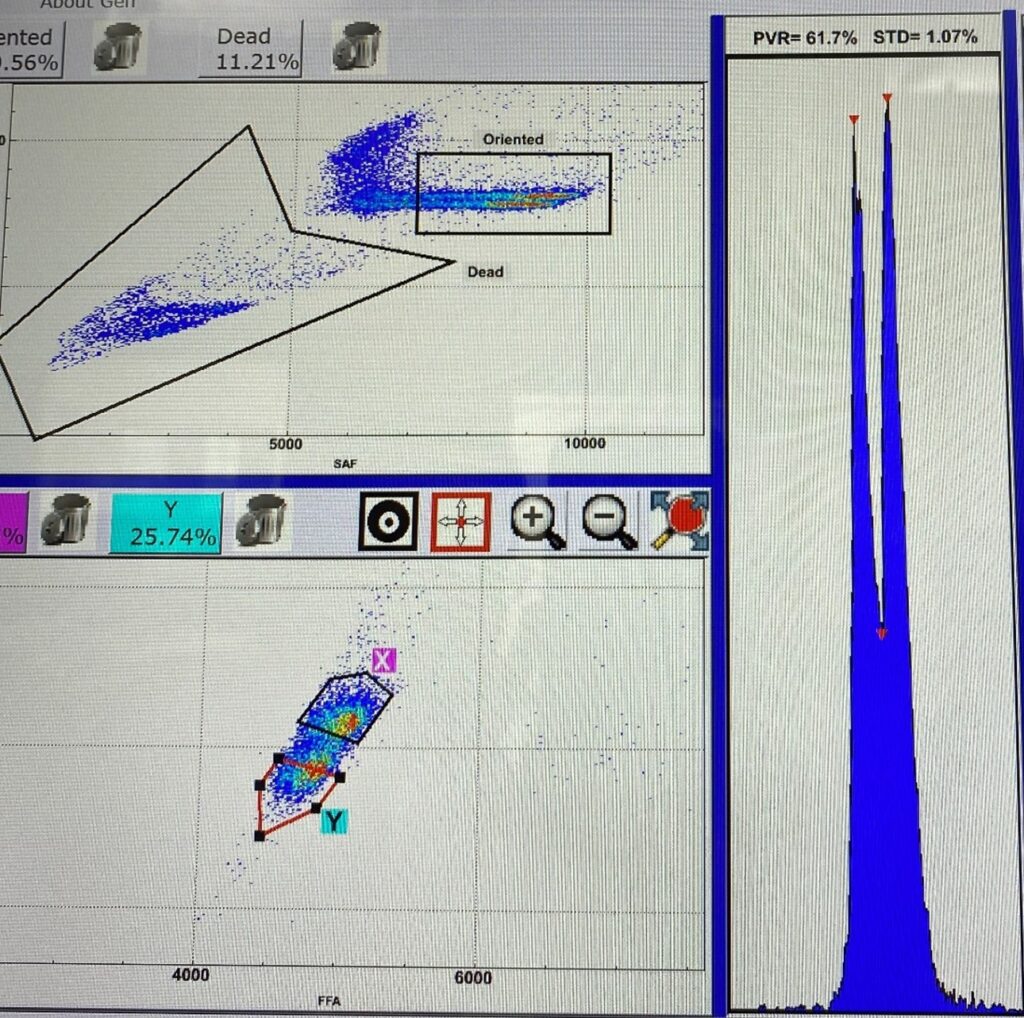
Canine sperm sexing, cryopreservation, and novel therapeutics to improve reproductive performance in working dog breeding programs
Sperm sexing technology is based on the difference in DNA content between X- and Y-chromosome-bearing sperm. This difference in DNA enables the separation of sperm into X- and Y-sperm populations by a modified flow cytometer and a fluorescent dye that specifically binds to DNA (Hoechst 33342). In collaboration with Sexing Technologies Genetics® in Texas, the sex sorting process for canine semen will be optimized. This innovative technology, when coupled with sperm cryopreservation and artificial insemination, facilitates rapid genetic gain in large scale working dog breeding programs. In our lab we are developing a canine semen freezing extender, incorporating low molecular weight cryoprotectants and cholesterol loading molecules to improve sperm membrane tolerance to the cryopreservation process. Simultaneously, the integration of prostatic fluid-derived extracellular vesicles into cryopreserved, sexed canine semen post-thawing is being explored. This initiative aims to augment viability and longevity within the female reproductive tract and ultimately improve reproductive performance particularly when utilizing the non-invasive transcervical insemination technique (TCI).
Contraception
Our goals are to:
- Demonstrate that extracellular vesicles (EVs), such as those produced by organoids, display tropism toward their tissue of origin and
- Establish gene editing by EVs loaded with CRISPR-cas9 ribonucleoproteins in vitro using an oviductal organoid model and in vivo in a mouse model. The findings of the proposed studies may lead to a novel, non-surgical, permanent contraceptive for dogs, cats, horses, and women.

EQUINE AND CANINE FETALLY-DERIVED MESENCHYMAL STEM CELLS (FDMSCS) AND THEIR EXTRACELLULAR VESICLES (EVS) AS POTENTIAL THERAPEUTICS FOR ENDOMETRITIS, OSTEOARTHRITIS and OCULAR DISEASES IN DOGS AND HORSES
MSCs derived from gestational tissues are more “plastic” and less antigenic than adult derived MSCs. Recent evidence suggests that EVs are responsible for the anti-inflammatory activity and enhancement of tissue regeneration that occurs with MSC treatment. Our lab has established and characterized both canine and equine late embryonic-derived MSCs lines and isolated and characterized EVs derived from these cells. These MSCs and their EVs are currently being implemented in a number of different therapeutic clinical trials being performed at CSU including persistent breeding induced endometritis (PBIE) in mares, endometritis in bitches, spontaneous chronic corneal epithelial defects in dogs, and recurrent uveitis in horses.
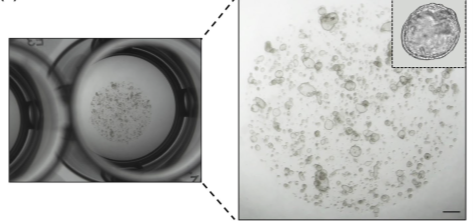
Bovine Oviductal Organoids in Assisted Reproductive Technologies
In collaboration with the Dawit Tesfaye lab, organoids will be utilized to study and recapitulate oviductal physiology in vitro, with the potential to produce EVs for downstream use in current IVF systems to improve the quality and quality of bovine blastocysts and ultimately pregnancy rates after embryo transfer.
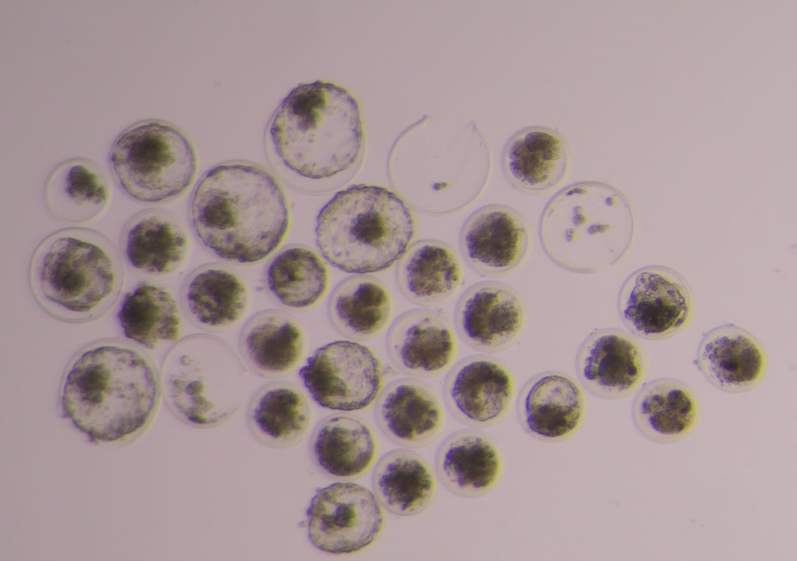
ADDITION OF BOVINE SEMINAL PLASMA DERIVED EVS TO FROZEN-THAWED BULL SPERM TO IMPROVE IN VITRO PRODUCTION OF EMBRYOS
Sex sorted bull sperm result in lower conception rates than unsorted sperm, regardless of whether the sperm are used for artificial insemination or in vitro fertilization (IVF) systems which is a huge economic loss to the dairy industry. In collaboration with Dr. James Graham, we are investigating the effect of EVs derived from bovine seminal plasma , oviductal fluid and oviductal organoids on frozen-thawed bovine sperm function and in vitro fertility